OCASCR funding has helped scientists purchase a variety of state-of-the-art pieces of equipment that are housed in different research institutions throughout Oklahoma. Equipment that is purchased in part or in full using OCASCR funds belongs to a virtual OCASCR Equipment Core and must be made available for use by any Oklahoma researcher. Individual pieces of OCASCR Core Equipment and their housing locations are listed below. Please contact the individuals associated with the equipment for information about accessibility, training (if required), and associated consumable and/or service fees.
 BD Biosciences’ LSRII cell analyzer was recently upgraded with funds from the Oklahoma Center for Adult Stem Cell Research (OCASCR). It now utilizes four lasers (407 nm, 488 nm, 561 nm and 633 nm air-cooled lasers) and precise optics to detect up to 16 independent signals. The 488 nm laser can be used to detect common fluorochromes including GFP, FITC and PerCP. The 561 nm laser is great for exciting PE and PE tandem dyes. The 633 nm laser is used to detect APC, APC-Cy7, APC-Cy5.5 and Cy5. These fluorochromes are most commonly used by our investigators who study hematopoietic stem cells and committed progenitors or B cell subsets from tonsils. Finally, the 407 nm laser is appropriate for FRET experiments, exciting Quantum Dots and for using Hoechst 33342 to study side population cells.Click here for more information.Location: Oklahoma Medical Research Foundation, Oklahoma City. Contact either Jacob Bass or Diana Hamilton at 405-271-7299.(Flow Cytometry Core Facility)
BD Biosciences’ LSRII cell analyzer was recently upgraded with funds from the Oklahoma Center for Adult Stem Cell Research (OCASCR). It now utilizes four lasers (407 nm, 488 nm, 561 nm and 633 nm air-cooled lasers) and precise optics to detect up to 16 independent signals. The 488 nm laser can be used to detect common fluorochromes including GFP, FITC and PerCP. The 561 nm laser is great for exciting PE and PE tandem dyes. The 633 nm laser is used to detect APC, APC-Cy7, APC-Cy5.5 and Cy5. These fluorochromes are most commonly used by our investigators who study hematopoietic stem cells and committed progenitors or B cell subsets from tonsils. Finally, the 407 nm laser is appropriate for FRET experiments, exciting Quantum Dots and for using Hoechst 33342 to study side population cells.Click here for more information.Location: Oklahoma Medical Research Foundation, Oklahoma City. Contact either Jacob Bass or Diana Hamilton at 405-271-7299.(Flow Cytometry Core Facility)
 Nikon Ti inverted motorized microscope with brightfield and fluorescence capability for slides and 96-well dish formats.Click here for more information. Location: University of Oklahoma Health Sciences Center, Oklahoma City. Contact Randy May at 405-824-6237 or Randal-may@ouhsc.edu.
Nikon Ti inverted motorized microscope with brightfield and fluorescence capability for slides and 96-well dish formats.Click here for more information. Location: University of Oklahoma Health Sciences Center, Oklahoma City. Contact Randy May at 405-824-6237 or Randal-may@ouhsc.edu.
Nikon Biostation live cell imager. Capable of imaging cells in multiple specified locations on 35mm glass dishes (brightfield and fluorescence) over hours or even days. Location: University of Oklahoma Health Sciences Center, Oklahoma City. Contact Randy May at 405-824-6237 or Randal-may@ouhsc.edu.
Capable of imaging cells in multiple specified locations on 35mm glass dishes (brightfield and fluorescence) over hours or even days. Location: University of Oklahoma Health Sciences Center, Oklahoma City. Contact Randy May at 405-824-6237 or Randal-may@ouhsc.edu.
Flexivent. The FlexiVent (http://www.scireq.com/products/flexivent/)is a combined computer-controlled piston ventilator and respiratory mechanics data acquisition platform. It measures respiratory mechanics and lung functions including resistance, elastance, compliance, and pressure-volume loop curves. The equipment is useful in assessing the efficacy of adult stem cell therapy of acute and chronic lung diseases. Location: Oklahoma State University, Stillwater, Oklahoma. Contact name: Lin Liu;
Contact #: 405-744-4526 or lin.liu@okstate.edu
 FLIM Systems for Zeiss LSM 710.This upgrade to the Zeiss LSM 710 multiphoton microscope includes a time-correlated single photon counting (TCSPC) system produced by Becker & Hickl GmbH. The instrument consists of: (i) Simple-Tau 152 TCSPC FLIM: a highly flexible, modular, dual channel parallel acquisition FLIM system; (ii) two HPM-100-40 GaAsP hybrid detector modules, with high count rate, fast response, no after pulsing and no diffusion tail, characteristics that make it perfect both for FLIM and FCS (fluorescence correlation spectroscopy); (iii) Data Analysis Software for Fluorescence Lifetime Imaging for Windows 95/98/NT/ME/2K/XP/VISTA. The system allows: (i) time-series FLIM as fast as 2 images / second; (ii) fluorescence correlation spectroscopy (FCS) recording, online-correlation and fit; (iii) Single, double, triple-exponential decay analysis; (iv) FRET measurement without bleedthrough; (v) double-exponential FRET for separation of interacting and non-interacting protein fractions; (vi)Ion concentration measurements.Click here for more information. http://imaging.omrf.org/microscopes/Location: Oklahoma Medical Research Foundation, Oklahoma City. Contact Ben Fowler at phone: (405) 271-7245 or (405) 271-7545 or ben-fowler@omrf.org.
FLIM Systems for Zeiss LSM 710.This upgrade to the Zeiss LSM 710 multiphoton microscope includes a time-correlated single photon counting (TCSPC) system produced by Becker & Hickl GmbH. The instrument consists of: (i) Simple-Tau 152 TCSPC FLIM: a highly flexible, modular, dual channel parallel acquisition FLIM system; (ii) two HPM-100-40 GaAsP hybrid detector modules, with high count rate, fast response, no after pulsing and no diffusion tail, characteristics that make it perfect both for FLIM and FCS (fluorescence correlation spectroscopy); (iii) Data Analysis Software for Fluorescence Lifetime Imaging for Windows 95/98/NT/ME/2K/XP/VISTA. The system allows: (i) time-series FLIM as fast as 2 images / second; (ii) fluorescence correlation spectroscopy (FCS) recording, online-correlation and fit; (iii) Single, double, triple-exponential decay analysis; (iv) FRET measurement without bleedthrough; (v) double-exponential FRET for separation of interacting and non-interacting protein fractions; (vi)Ion concentration measurements.Click here for more information. http://imaging.omrf.org/microscopes/Location: Oklahoma Medical Research Foundation, Oklahoma City. Contact Ben Fowler at phone: (405) 271-7245 or (405) 271-7545 or ben-fowler@omrf.org.
 ImageXpress Micro High Content Screening System.Automated imaging acquisition for stem cell-based high throughput screening.Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact-Contact-Ben Fowler (405) 271-7245 or Ben-Fowler@omrf.org.
ImageXpress Micro High Content Screening System.Automated imaging acquisition for stem cell-based high throughput screening.Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact-Contact-Ben Fowler (405) 271-7245 or Ben-Fowler@omrf.org.
 FluoView FV1000.Follow-up high resolution imaging acquisition of high throughput screening. Click here for more information.Location-Oklahoma Medical Research Foundation, Oklahoma City. Contact-Contact-Ben Fowler (405) 271-7245 or Ben-Fowler@omrf.org.
FluoView FV1000.Follow-up high resolution imaging acquisition of high throughput screening. Click here for more information.Location-Oklahoma Medical Research Foundation, Oklahoma City. Contact-Contact-Ben Fowler (405) 271-7245 or Ben-Fowler@omrf.org.
 Bioscope Catalyst Atomic force microscope.The Bioscope Catalyst can perform four types of studies. (1) High-resolution, molecular-scale imaging of biological samples such as lipid bilayers and membranes, DNA-plasmid and other biomolecules, and biopolymer structures. (2) Live cell imaging for studying structure and function relationships, and cell response to environmental stimuli. (3) Force and mechanical studies of cells such as cell membrane elasticity, mapping ligand-receptor interactions, and single-molecule force spectroscopy. (4) Nanomanipulation of biomolecules. This equipment can image biological samples in both wet and dry states and at both lower and higher temperature. Location- University of Oklahoma, Norman Campus. Contact Yiton Dong yiton.dong-1@ou.edu
Bioscope Catalyst Atomic force microscope.The Bioscope Catalyst can perform four types of studies. (1) High-resolution, molecular-scale imaging of biological samples such as lipid bilayers and membranes, DNA-plasmid and other biomolecules, and biopolymer structures. (2) Live cell imaging for studying structure and function relationships, and cell response to environmental stimuli. (3) Force and mechanical studies of cells such as cell membrane elasticity, mapping ligand-receptor interactions, and single-molecule force spectroscopy. (4) Nanomanipulation of biomolecules. This equipment can image biological samples in both wet and dry states and at both lower and higher temperature. Location- University of Oklahoma, Norman Campus. Contact Yiton Dong yiton.dong-1@ou.edu
 TE10 Automatic Cigarette Smoke System.The Teague Enterprises cigarette whole body exposure system consists of a method for smoking, metering and measuring smoke delivered to exposure chambers. The smoking machine can smoke from one to ten cigarettes at a time using the FTC method of puffing i.e. a puff of 35 cc for two seconds once a minute. The operator can choose the smoke concentration by varying the number of cigarettes and the dilution air allowed to mix with the smoke before entering the exposure chambers. Smoke from the machine is collected from the side stream and mixed with the mainstream smoke where it is collected in the aging and dilution chamber where it is mixed with a fan. The diluted smoke is then metered into the exposure chambers for exposures. The smoke from the chambers is vented through an activated charcoal filter and discarded into the building exhaust. The chambers can be used together or one can be used as control while the other for smoke exposure. The smoking machine is located in a specially designed hood that captures the errant smoke to keep it out of the smoking system area. The hood contains the exhaust fan metering devices and compressor used to operate the smoking machine. The system also includes a filter holder, vacuum pump and gas meter for measuring the TSP (Total Suspended Particulate).Click here for more information. Location- Oklahoma State University, Stillwater, OK. Contact Dr. Lin Liu- lin.liu@okstate.edu
TE10 Automatic Cigarette Smoke System.The Teague Enterprises cigarette whole body exposure system consists of a method for smoking, metering and measuring smoke delivered to exposure chambers. The smoking machine can smoke from one to ten cigarettes at a time using the FTC method of puffing i.e. a puff of 35 cc for two seconds once a minute. The operator can choose the smoke concentration by varying the number of cigarettes and the dilution air allowed to mix with the smoke before entering the exposure chambers. Smoke from the machine is collected from the side stream and mixed with the mainstream smoke where it is collected in the aging and dilution chamber where it is mixed with a fan. The diluted smoke is then metered into the exposure chambers for exposures. The smoke from the chambers is vented through an activated charcoal filter and discarded into the building exhaust. The chambers can be used together or one can be used as control while the other for smoke exposure. The smoking machine is located in a specially designed hood that captures the errant smoke to keep it out of the smoking system area. The hood contains the exhaust fan metering devices and compressor used to operate the smoking machine. The system also includes a filter holder, vacuum pump and gas meter for measuring the TSP (Total Suspended Particulate).Click here for more information. Location- Oklahoma State University, Stillwater, OK. Contact Dr. Lin Liu- lin.liu@okstate.edu
 Accuri C6 Flow Cytometer.Flow cytometry is a fluorescence-based technique in which cells in solution are passed through a laser beam and the fluorescence and light scattering characteristics of cells are measured. This technique allows for the study of a variety of important cell parameters including: cell volume and morphological complexity, cell surface molecules (e.g. membrane proteins, antigens, & phospholipids), intracellular molecules (e.g. Calcium levels, DNA & RNA content), intracellular pH, cell viability, and cell apoptosis. Because of these unique and extensive capabilities, multi-color flow cytometry is one of the most powerful and critical instruments for cell characterization and research. We have purchased a BD Accuri C6 flow cytometer and the associated computer workstation bundle. The BD Accuri C6 is equipped with (i) two lasers which will allow for blue (488 nm) and red (633 nm) excitation, (ii) two light scatter detectors (i.e. forward & side scatter) and (iii) four fluorescence detectors (3 blue: 533 nm, 585 nm, 670 nm; 1 red: 675 nm). Two unique features of the BD Accuri C6 are that (i) it has a non-pressurized fluidics system which allows for continuous monitoring of the effects of test compounds (e.g. calcium modulators) on cell behavior; and (ii) it is capable of acquiring 7 decades of dynamic range (i.e. 16 million channels of digital data). This large range eliminates the need for adjustable gain settings on the detectors, which reduces setup time and the consumption of both sheath fluid and sample volume. By collecting seven decades of dynamic range, all the time, instead of the traditional four, all the data is recorded all the time, and no data is ever lost.Click here for more information. Location- University of Oklahoma – Norman Campus. Contact Dr. Vassilios Sikavitsas – vis@ou.edu
Accuri C6 Flow Cytometer.Flow cytometry is a fluorescence-based technique in which cells in solution are passed through a laser beam and the fluorescence and light scattering characteristics of cells are measured. This technique allows for the study of a variety of important cell parameters including: cell volume and morphological complexity, cell surface molecules (e.g. membrane proteins, antigens, & phospholipids), intracellular molecules (e.g. Calcium levels, DNA & RNA content), intracellular pH, cell viability, and cell apoptosis. Because of these unique and extensive capabilities, multi-color flow cytometry is one of the most powerful and critical instruments for cell characterization and research. We have purchased a BD Accuri C6 flow cytometer and the associated computer workstation bundle. The BD Accuri C6 is equipped with (i) two lasers which will allow for blue (488 nm) and red (633 nm) excitation, (ii) two light scatter detectors (i.e. forward & side scatter) and (iii) four fluorescence detectors (3 blue: 533 nm, 585 nm, 670 nm; 1 red: 675 nm). Two unique features of the BD Accuri C6 are that (i) it has a non-pressurized fluidics system which allows for continuous monitoring of the effects of test compounds (e.g. calcium modulators) on cell behavior; and (ii) it is capable of acquiring 7 decades of dynamic range (i.e. 16 million channels of digital data). This large range eliminates the need for adjustable gain settings on the detectors, which reduces setup time and the consumption of both sheath fluid and sample volume. By collecting seven decades of dynamic range, all the time, instead of the traditional four, all the data is recorded all the time, and no data is ever lost.Click here for more information. Location- University of Oklahoma – Norman Campus. Contact Dr. Vassilios Sikavitsas – vis@ou.edu
 Spinning disk confocal microscope for intravital imaging.The intra-vital microscopy system is based on the Yokogawa Corp.’s CSX M1-L scanning disk upgraded to 5000 rpm (1000 scan/sec). The system allows high speed image acquisition at high resolution and with low photo-toxicity. This system has an AOTF controlled laser array providing 405, 488, 514, 568, 647 nm laser lines. A custom computer drives a stage with piezo 300 µmZ axis control for acquisition of multi-color Z stacks. The system includes the following emission band pass or long pass filters (Chroma): 460/50, 525/50, 605/52, 700/75. These filters allow the detection of the following fluorophores: DAPI-CFP/GFP, FITC/mCherry/Cy5. For image acquisition, the system includes a Hamamatsu EMCCD 512×512 driven by Micro-manager 1.4 open source software. The whole system is controlled by a WIN 7 Imaging Quad-core workstation with 12 GB RAM, 1 TB HD, and a 24″ FP monitor.Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact- Dr. Tadayuki Yago@omrf.org and Dr. Zhenghui Liu Zhenghui-Liu@omrf.org
Spinning disk confocal microscope for intravital imaging.The intra-vital microscopy system is based on the Yokogawa Corp.’s CSX M1-L scanning disk upgraded to 5000 rpm (1000 scan/sec). The system allows high speed image acquisition at high resolution and with low photo-toxicity. This system has an AOTF controlled laser array providing 405, 488, 514, 568, 647 nm laser lines. A custom computer drives a stage with piezo 300 µmZ axis control for acquisition of multi-color Z stacks. The system includes the following emission band pass or long pass filters (Chroma): 460/50, 525/50, 605/52, 700/75. These filters allow the detection of the following fluorophores: DAPI-CFP/GFP, FITC/mCherry/Cy5. For image acquisition, the system includes a Hamamatsu EMCCD 512×512 driven by Micro-manager 1.4 open source software. The whole system is controlled by a WIN 7 Imaging Quad-core workstation with 12 GB RAM, 1 TB HD, and a 24″ FP monitor.Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact- Dr. Tadayuki Yago@omrf.org and Dr. Zhenghui Liu Zhenghui-Liu@omrf.org
 LSM 710; Laser Scanning Confocal Microscope.The LSM710 confocal microscope is an environmentally controllable system (CO2, temperature, humidity) useful for live and fixed sample imaging. Six unique excitation wavelengths (covering UV to far red) and a 36 array detection system allows for examination of multiple fluorphores both simultaneously and sequentially. Definite focus and z-plane acquisition parameters add increased functionality allowing for imaging experiments to proceed unattended for multiple days, and the reduction of background fluorescence in thicker samples, respectively. The user-friendly Zen software platform also allows for FRET, FRAP, and Mosaic Imagining.Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact- Ben Fowler (405) 271-7245. href=”mailto:Ben-Fowler@omrf.org”>Ben-Fowler@omrf.org
LSM 710; Laser Scanning Confocal Microscope.The LSM710 confocal microscope is an environmentally controllable system (CO2, temperature, humidity) useful for live and fixed sample imaging. Six unique excitation wavelengths (covering UV to far red) and a 36 array detection system allows for examination of multiple fluorphores both simultaneously and sequentially. Definite focus and z-plane acquisition parameters add increased functionality allowing for imaging experiments to proceed unattended for multiple days, and the reduction of background fluorescence in thicker samples, respectively. The user-friendly Zen software platform also allows for FRET, FRAP, and Mosaic Imagining.Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact- Ben Fowler (405) 271-7245. href=”mailto:Ben-Fowler@omrf.org”>Ben-Fowler@omrf.org
 FacsAria IIu.This upgrade allows for greater stability in the fluidics used by the instrument and more stability during the process of sorting cells. The upgrade utilizes a simplification of the fluidics that will help decrease the downtime required for service calls. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact- Jacob Bass or Diana Hamilton. (Flow Cytometry Core Facility)
FacsAria IIu.This upgrade allows for greater stability in the fluidics used by the instrument and more stability during the process of sorting cells. The upgrade utilizes a simplification of the fluidics that will help decrease the downtime required for service calls. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact- Jacob Bass or Diana Hamilton. (Flow Cytometry Core Facility)
 Olympus SZX16 with fluorescence, transmitted light stand and high resolution digital camera.
Olympus SZX16 with fluorescence, transmitted light stand and high resolution digital camera.
Central to the analyses of stem cell characteristics (stem cell proliferation and differentiation) in intact tissues is the ability to perform dissections of small animals followed by the visualization of transgene expression in the adult stem cells of the different organs. For instance, by genetically marking the midgut stem cells of the adult fruitfly, Drosophila melanogaster, with the green fluorescent protein (GFP), one can utilize this equipment to perform convenient dissection of the midgut and examine the GFP-expressing stem cells in the intact organ under fluorescence for any alterations in their number and size, which would be indicative of changes in adult stem cell division and differentiation. Moreover, with the ability to perform dissection and stem cell visualization with ease and convenience using this equipment, one can now conduct genetic screenings of novel genes that might regulate the regenerative capacity of somatic stem cells in the midgut (and other organs) of the adult fly during basal tissue turnover and in response to insults. Location-Oklahoma Medical Research Foundation, Oklahoma City.Contact- Contact- Ben Fowler (405) 271-7245 or Ben-Fowler@omrf.org
 MoFlo XDP.The upgrade brings the Moflo to a digital platform with a new XDP computer, new PMT detectors, new software and a touch screen panel to interface with the XDP electronics.Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact- Jacob Bass or Diana Hamilton. (Flow Cytometry Core Facility).
MoFlo XDP.The upgrade brings the Moflo to a digital platform with a new XDP computer, new PMT detectors, new software and a touch screen panel to interface with the XDP electronics.Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact- Jacob Bass or Diana Hamilton. (Flow Cytometry Core Facility).
 Microm HM 355 Microtome.This rotary microtome will be used for sectioning paraffin blocks of a variety of tissues from transgenic mice for immunohistochemistry and H&E staining. Both structural and antigen differences will be analyzed and quantified on projects studying colitis and vascular development. This equipment uses low profile disposable blades with the capability of cutting 0.5-100µm sections in 0.5µm below 5µm and 1µm increments above 5µm. A trimming function is also present from 5-250µm. Horizontal feed and retraction movements are carried out by a moving knife carrier. Foot control is also present as an option.Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact- Michael McDaniel- 405-271-3979
Microm HM 355 Microtome.This rotary microtome will be used for sectioning paraffin blocks of a variety of tissues from transgenic mice for immunohistochemistry and H&E staining. Both structural and antigen differences will be analyzed and quantified on projects studying colitis and vascular development. This equipment uses low profile disposable blades with the capability of cutting 0.5-100µm sections in 0.5µm below 5µm and 1µm increments above 5µm. A trimming function is also present from 5-250µm. Horizontal feed and retraction movements are carried out by a moving knife carrier. Foot control is also present as an option.Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact- Michael McDaniel- 405-271-3979
 InsituPro VSI.Even though adult stem cells are very rare, they can be detected in normal and diseased tissues by immunohistochemistry (IHC) using distinct protein markers, and by in situ hybridization (ISH) using unique DNA/RNA expression profile. Thus, IHC/ISH can be a powerful tool to visualize stem cells undergoing programming and reprogramming in tissue regeneration and to better understand tissue-specific stem cells, which are two research areas of particular interest to OCASCR. Similarly, IHC/ISH is a valuable tool in identifying misfated cancer stem cell (CSC) number and distribution in solid tumors such as breast, prostate, and colorectal cancers. Analysis of specific markers in many, rare occurring cells, however, is often limited by the throughput and low complexity of the analysis technique. An improved understanding of rare stem cells will come from high-throughput multiplexed IHC/ISH, using human tissue samples or model organisms, which can be used to study the role of a specific gene (by knockout or over-expression) in CSCs or evaluate the therapeutic value of candidate compounds against this specific cancerous subpopulation. Finally, high-throughput ISH/IHC is also compatible with large-scale gene expression profiling studies (e.g. microarray, whole genome sequencing) to rapidly obtain detailed expression levels of candidate genes at the cellular, tissue, or whole organism level. In situ detection of DNA, RNA and proteins are powerful techniques to gain data about the temporal and spatial expression pattern of genes within complete organisms. The Vsi can performed this tedious process on whole mounts, vibratome sections or thin sections fixed on slides. Our instruments automate most steps of these tedious and labor-intensive methods. Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact- Guarev Varshney, Ph.D.- 405-271-2185 or Gaurav-Varshney@omrf.org.
InsituPro VSI.Even though adult stem cells are very rare, they can be detected in normal and diseased tissues by immunohistochemistry (IHC) using distinct protein markers, and by in situ hybridization (ISH) using unique DNA/RNA expression profile. Thus, IHC/ISH can be a powerful tool to visualize stem cells undergoing programming and reprogramming in tissue regeneration and to better understand tissue-specific stem cells, which are two research areas of particular interest to OCASCR. Similarly, IHC/ISH is a valuable tool in identifying misfated cancer stem cell (CSC) number and distribution in solid tumors such as breast, prostate, and colorectal cancers. Analysis of specific markers in many, rare occurring cells, however, is often limited by the throughput and low complexity of the analysis technique. An improved understanding of rare stem cells will come from high-throughput multiplexed IHC/ISH, using human tissue samples or model organisms, which can be used to study the role of a specific gene (by knockout or over-expression) in CSCs or evaluate the therapeutic value of candidate compounds against this specific cancerous subpopulation. Finally, high-throughput ISH/IHC is also compatible with large-scale gene expression profiling studies (e.g. microarray, whole genome sequencing) to rapidly obtain detailed expression levels of candidate genes at the cellular, tissue, or whole organism level. In situ detection of DNA, RNA and proteins are powerful techniques to gain data about the temporal and spatial expression pattern of genes within complete organisms. The Vsi can performed this tedious process on whole mounts, vibratome sections or thin sections fixed on slides. Our instruments automate most steps of these tedious and labor-intensive methods. Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact- Guarev Varshney, Ph.D.- 405-271-2185 or Gaurav-Varshney@omrf.org.
 Wes
Wes
Add your samples and primary antibody or labeling reagent to the pre-filled assay plate. Pop in Wes’™ capillary cartridge and load the plate. Push start and in just 3 hours get quantitated size-based separation data on up to 25 samples. After the run, toss the used cartridge and plate and you’re done.
Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact- Magdalena Bieniasz, Ph.D. Magdalena-Bieniasz@omrf.org.
.
 Light Cycler 480
Light Cycler 480
The LightCycler® 480 System is a high-performance, medium- to high-throughput PCR platform (96- or 384-well plates) that provides various methods for gene detection, gene expression analysis, genetic variation analysis, and array data validation. The system features the LightCycler® 480 Instrument, a versatile, plate-based real-time PCR device that supports mono- or multicolor applications, as well as multiplex protocols. The benchtop instrument is easily customizable to meet changing user requirements, and can be integrated into everyday use as a robotically controlled, automated high-throughput solution. The LightCycler® 480 Instrument is designed for general laboratory use and is not intended for use in diagnostic procedures. Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact-Duane Goins- 405-271-5558 or Duane-Goins@omrf.org.

The microinjection system and stereo microscopes in tandem will be used to inject genetic material into the zebrafish embryo. The core utilizes knockout technology in zebrafish (Danio rerio) as a simple vertebrate model system and an economical alternative for high throughput analysis of stem cell programming and reprogramming in juxtaposition with lower throughput mouse models system. The zebrafish is ideal for stem cell studies because of its rapid embryonic development that occurs ex utero, the transparency of embryos, and the large clutch sizes obtained from zebrafish mating. Thus, it is ideal for large scale, multiparametric studies as well as high-throughput screens. Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact- Guarev Varshney, Ph.D.- 405-271-2185 or Gaurav-Varshney@omrf.org.
![OMX SR system-3[1]](http://www.ocascr.org/wp-content/uploads/2016/07/OMX-SR-system-31.jpg)
The DeltaVision OMX SR is a compact imaging system that is specifically optimized to provide a stable platform for Structured Illumination Microscopy (SIM) super resolution technology.
- Super-resolve live cells: 2D SIM and 2D SIM-TIRF imaging modes allow for dynamic super-resolution live-cell imaging.
- Molecular imaging: Localization microscopy option utilizes exclusive 2D multi-emitter fitting algorithms to reconstruct a super-resolved image.
- More data from your sample: Multiplex data acquisition with simultaneous multi-camera imaging.
- Preserve cell viability: Ultra-low light imaging combined with advanced deconvolution algorithm.
- Capture dynamic processes: Rapid image acquisition and stage automation.
Click here for more information. Location- Oklahoma Medical Research Foundation, Oklahoma City. Contact-Ben Fowler (405) 271-7245 or Ben-Fowler@omrf.org.
The Leica LMD7000 is based on a fully automated upright research microscope. The LMD 7000 is the first upright laser dissection system, using gravity as the means of capturing the dissected materials. This allows for the sample (nucleus, single cell, or small selected area) to be cut and dropped into a capture cap with different sizes of array setting as 4, 8, 16, or 48 arrays. Array tubes can be loaded with buffer to preserve the dissectate, allowing more time to collect a greater number of samples. Gravity based sample collection allows for contamination free sample collection. Large samples be collected into the tube (even entire slides) with no shape or size limit. The LMD7000 uses a 349 nm laser which allows control over the frequency and pulse duration (120 J at up to 5000 Hz). This translates to more power, specificity, and bandwidth for samples that are difficult to cut, such as bone, without heating samples. Four fluorescent colors (blue, green, red, and far red) are available. Contact-Muralidharan Jauaraman, Ph.D.(405) 271-8001 Ext. 30492 or Muralidharan-Jayaraman@ouhsc.edu
The In-Vivo Xtreme II™ provides comprehensive multimodal optical imaging that supports the broadest range of research objectives of any preclinical optical imaging system. Five imaging modalities are provided, with co-registration of Bioluminescence Imaging (BLI), Multispectral VIS-NIR Fluorescence Imaging (MS-FLI), unique Direct Radioisotopic Imaging (DRI), Cherenkov Imaging (CLI), and X-ray Imaging. Click here for more information. Location-Center for Veterinary Health Sciences, 004 E McElroy Hall, Oklahoma State University, Stillwater Contact:Ashish.ranjan@okstate.edu or Myron.Hinsdale@okstate.edu 405-744-6292.
 Leica TCS SP* Laser Scanning Confocal Microscope Less phototoxicity and more viability for developing organisms. All optical components of the Leica TCS SP8 are matched to preserve photons for brilliant images full of contrast. With the synergies of the multiband spectral detector, acousto-optical beam splitter (AOBS), and super-sensitive Leica HyDs you get a confocal microscope with maximum photon efficiency and gapless spectral detection. This superior sensitivity directly translates into reduced laser power for cell viability and resolution. The Leica TCS SP8 platform offers advanced possibilities for gentle imaging of sensitive samples.Multiphoton (MP) imaging reduces out-of-focus excitation and increases the viability of your samples. Light sheet imaging with the Leica TCS SP8 DLS offers low illumination and high-speed acquisition, which reduces phototoxic effects and allows you to follow developing organisms over time. Location- University of Oklahoma Health Sciences Center Flow Cytometry and Imaging Facility. Contact: Dr. Allison Gillaspy (405-271-2337, allison-gillaspy@ouhsc.edu) or Dr. Jim Henthorn (405-271-2035 ext 12035, jim-hethorn@ouhsc.edu).
Leica TCS SP* Laser Scanning Confocal Microscope Less phototoxicity and more viability for developing organisms. All optical components of the Leica TCS SP8 are matched to preserve photons for brilliant images full of contrast. With the synergies of the multiband spectral detector, acousto-optical beam splitter (AOBS), and super-sensitive Leica HyDs you get a confocal microscope with maximum photon efficiency and gapless spectral detection. This superior sensitivity directly translates into reduced laser power for cell viability and resolution. The Leica TCS SP8 platform offers advanced possibilities for gentle imaging of sensitive samples.Multiphoton (MP) imaging reduces out-of-focus excitation and increases the viability of your samples. Light sheet imaging with the Leica TCS SP8 DLS offers low illumination and high-speed acquisition, which reduces phototoxic effects and allows you to follow developing organisms over time. Location- University of Oklahoma Health Sciences Center Flow Cytometry and Imaging Facility. Contact: Dr. Allison Gillaspy (405-271-2337, allison-gillaspy@ouhsc.edu) or Dr. Jim Henthorn (405-271-2035 ext 12035, jim-hethorn@ouhsc.edu).
 Nikon C3 Laser Scanning Confocal Microscope
Nikon C3 Laser Scanning Confocal Microscope
This inverted microscope is equipped with:
- A four-wavelength laser scanning confocal head that allows irradiation and ablation in any pattern desired (405, 488, 561, 640 nm lasers).
- Three PMT-based detectors that allow simultaneous acquisition of images at four wavelengths.
- Perfect Focus 3, a long-wave infrared system for Z positioning of objectives relative to the sample/dish interface to maintain focus over extended time periods (hours). An added benefit is that this system also allows the use of red-shifted dyes.
- A motorized stage to allow simultaneous time-lapse acquisition of data from multiple samples.
- A workstation equipped with the intuitive NIS Elements C software for data acquisition, processing and analysis.
- Objective lenses: 10x, 20x, and 60X
- Ex-Cite LED epi-illumination for visual scanning of samples.
Location: Oklahoma Medical Research Foundation Contact: Susannah-Rankin@omrf.org
Illumna ddSEQ
The ddSEQ Single-Cell Isolator is part of the Illumina® Bio-Rad® Single-Cell Sequencing Solution, a platform that brings Bio-Rad’s Droplet Digital™ technology and Illumina’s market-leading sequencing expertise to single-cell gene expression studies.
The ddSEQ Single-Cell Isolator works with the Illumina® Bio-Rad® SureCell™ WTA 3′ Library Prep Kit for the ddSEQ™ System. This technology utilizes disposable microfluidic cartridges to coencapsulate single cells and barcodes into subnanoliter droplets, where cell lysis and barcoding occur. RNA-Seq libraries are subsequently prepared and sequenced. Analysis is conducted via BaseSpace. Click here for more information.
Location: University of Oklahoma Health Sciences Center. Contact: Ben Wronowski, Benjamin-Wronowski@ouhsc.edu -(405)271-8000 ext. 30477
BioSpherix Hypoxia Culture system with Buffer Chamber, Processing Chambers, and Direct Heat Incubator
The BioSpherix “c-shuttle hypoxia hood” system is an adaptable system for conducting cell and tissue culture experiments in hypoxia. The system includes a hypoxia culture glovebox that allows users to maintain the hypoxic oxygen levels while working with biological samples. Three independent hypoxia culture chamber-sensor units (“c-chambers”) are micro-chambers that are placed inside a dedicated incubator. Each “c-chamber” can be independently controlled to the desired micro-environment oxygen and carbon dioxide levels during extended culture. Location: Oklahoma Medical Research Foundation.Contact: Tim-Griffin@omrf.org 405-271-7579
Nanosight NS300
NanoSight instruments provide data on particle size distribution, concentration and aggregation, at extremely high resolution. Particles contained in a sample are visualized by virtue of the light they scatter when illuminated by laser light. The light scattered by the particles is captured using a scientific digital camera and the motion of each particle is tracked from frame to frame by the specially developed Nanosight software. This type of tracking produces a number weighted particle size distribution and provides concentration in particles/ml for each size family detected. Virtually any nanomaterial system with a particle size greater than 40nm can be characterized by NanoSight including exosomes, vesicles, virus (many types), virus like particles, inorganic nanoparticles, quantum dots, liposomes, solid lipid nanoparticles and other drug delivery systems. The addition of a fluorescence option dramatically extends the capabilities of the instruments, allowing truly multi-parametric characterization of nanoparticles through their size, intensity, and fluorescent signature to confirm specificity in the tracking.
Location: Oklahoma State University- Stillwater Contact: Lin Liu Lin.liu@okstate.edu (405)744-4526 or Yurong Liang yurongl@okstate.edu (405)744-1686
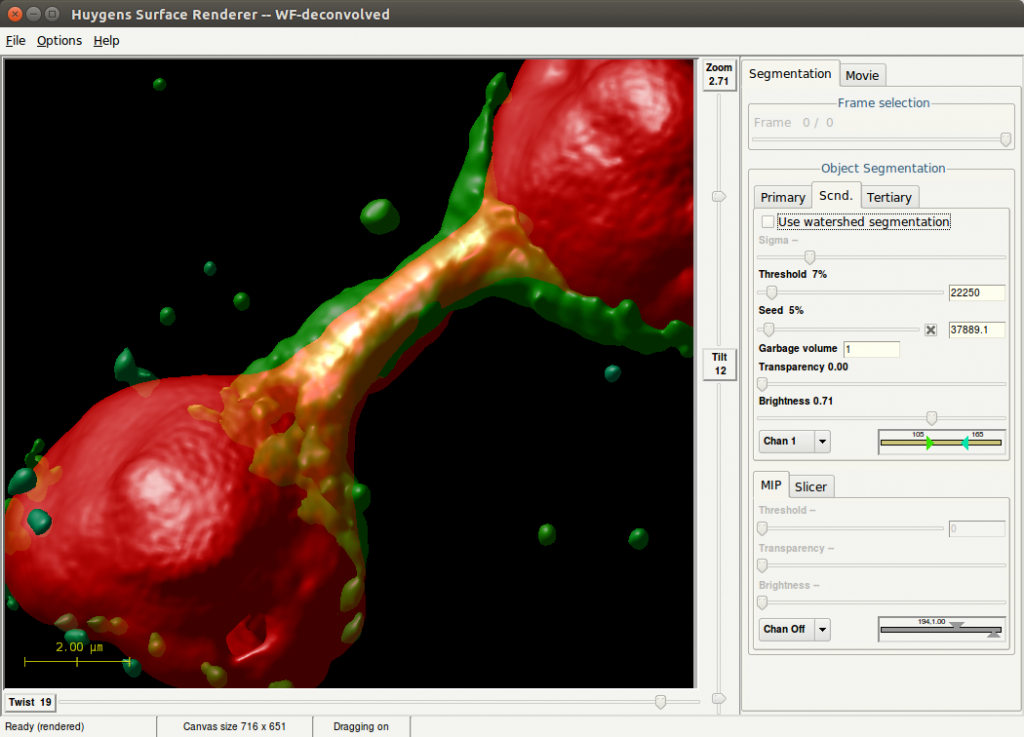 Computational Deconvolution Microscopy Workstation
Computational Deconvolution Microscopy Workstation
Huygens Essential is part of the Huygens Software, tailored for deconvolution and processing of microscopy images. Its wizard-driven user interface guides you through the process of deconvolving microscopy images. Huygens Essential is able to deconvolve a wide variety of images ranging from 2D widefield images to 4D Multi Channel multi-photon, spinning-disk, confocal, STED or light-sheet images. Comparison views of raw and deconvolved results is easily performed with the dual 4D slicer tool, and powerful and fast renderers produce high quality representations of your data. Based on the same image processing engine as Huygens Professional, Huygens Essential combines the quality and speed of the algorithms available in Huygens Professional with the ease of use of a wizard-driven intelligent user interface. Also, Huygens Essential is based on cross-platform technology which makes it available on a large range of platforms.
Location: Oklahoma Medical Research Foundation Contact: For software: Matthew Mendez, Ph.D matthew-menendez@omrf.org 405-271-7203
For hardware: Todd Walker Todd-walker@omrf.org405-271-7113
Gryphon protein crystallization robot with LCP adaptor for membrane proteins
The Crystal Gryphon is a small, fast and affordable option for setting up all of your crystal plates. The Crystal Gryphon fits into tight lab spaces and tight lab budgets.The 96-channel positive displacement head quickly fills screen reservoirs and protein wells. The head has no disposable tips, so the cost of operation and environmental waste is minimized.The 1-channel non-contact dispenser quickly aspirates and dispenses your proteins allowing your plate to be sealed without evaporation being a problem. This dispense head is designed specifically to handle proteins, features flow-through washing and positive displacement aspiration, to handle all of your proteins.Location: University of Oklahoma Health Sciences Center Contact: Dr. Simon Terzyan. Simon-terzyan@ouhsc.edu 405-271-8313
 GentleMACS Octo Dissociator with heaters
GentleMACS Octo Dissociator with heaters
The gentleMACS™ Octo Dissociator with Heaters is a benchtop instrument for the fully automated and standardized tissue dissociation or homogenization of up to eight samples. Equipped with eight individual heating units and numerous optimized and ready-to-use gentleMACS Programs the gentleMACS Octo Dissociator with Heaters offers a fully automated workflow for tissue dissociation. In addition the instrument allows the user to create user-defined programs for almost any biological material. All eight positions can be operated independently. Single-cell suspensions or thorough homogenates are easily and reproducibly obtained using the unique C Tubes or M Tubes. These single-use gentleMACS Tubes allow sample preparation in a closed and sterile system, providing a high level of user safety and minimize cross-contamination. Location- University of Oklahoma Health Sciences Center Contact: Ben Wronowski Benjamin-Wronowski@ouhsc.edu 405-271-8000 Ext. 30477
Scanning Electron Microscope (SEM)
The latest in benchtop SEM technology, the JCM-6000Plus “NeoScopeTM,” is a touch panel controlled, multi functional desktop scanning microscope that answers the increasingly diversified needs among users worldwide. The JCM-6000Plus is equipped with the high-sensitivity semiconductor detectors found in high-end instruments, making it easy to acquire composition contrast information about the specimen, and enabling efficient analysis. The series continues to include the high-vacuum functionality and secondary electron detector, offering the ability to clearly observe fine structures on the specimen surface at high magnification.
- Automatic image formation after sample introduction within 3 minutes
- High resolution (60,000X) and large depth of field
- Multi-touch screen interface for intuitive operation
- Advance automatic functions (focus, stigmation, brightness/contrast)
Location: Oklahoma Medical Research Foundation Contact: Sathish Srinivasan, Ph.D. Sathish-srinivasan@omrf.org 405-271-3550
 Nikon Ti2-E Inverted epifluorescence microscope
Nikon Ti2-E Inverted epifluorescence microscope
This is a state of the art microscope that will allow fluorescence image acquisition with minimal damage to the sample. This is achieved by having the light source “triggered” by the camera, minimizing exposure time, and thus enhancing the ability to collect time-lapse images without damaging sensitive live samples. In addition, this system is equipped with a sophisticated auto focusing system, and excitation and emission systems to allow imaging of many different fluorophores.
Location: Oklahoma Medical Research Foundation Contact: Susannah Rankin, Ph.D.-susannah-rankin@omrf.org-405-271-8190
Zeiss LSM 880 Laser Scanning Confocal Microscope
This point scanning confocal microscope is equipped with 6 laser lines and is configured with temperature and CO2 controls for long time-lapse imaging. It is also equipped with Zeiss proprietary Airy Scan detector, capable of super resolution imaging in real time.
Location: Oklahoma Medical Research Foundation Contact: Contact- Contact- Ben Fowler (405) 271-7245 or Ben-Fowler@omrf.org.
Leica M205-MFC THUNDER 2D/3D Large specimen imaging system
The Leica M205-MFC THUNDER 2D/3D Large specimen imaging system is equipped for imaging of large tissue specimens at magnifications of 1X to 32X. Specimens can be imaged and reconstructed in 2 or 3 dimensions using white light illumination or up to four fluorescence channels (Blue, Green, Near Red, Deep Red). The THUNDER image processing software package permits “computational clearing” of large specimens to reveal fluorescent labeling by removing background fluorescence without the need for chemical clearing of the tissue. The system also will support imaging of chemically-cleared fluorescent specimens. This is a very flexible imaging system that excels for imaging of large specimens, especially for multifluorescent analysis. Examples of commonly imaged specimens include: Retinal wholemounts and brain slices that have been immunolabeled or express fluorescent reporter proteins. Tissue sections from large specimens such as brain that have been histologically stained or immunolabeled.
Location: OUHSC Contact: David Sherry david-sherry@ouhsc.edu 405-271-2377 ext 45514
Gas Chromatography – Tandem Mass Spectrometry
(GC-MS/MS)
This combination of GC and MS is set to perform selective ion monitoring (SIM) for highly sensitivity measurements of stable isotopic enrichment. It can used with a variety of isotopes to answer many questions related to flux and synthesis. When operated in SIM mode, it facilitates metabolite detection and can be used for targeted metabolomics with the appropriate method development.
Location: OMRFContact: Benjamin Miller, Ph.D. Benjamin-miller@omrf.org 405-271-7767
OptoMotry HD system
This non-invasive, quantitative tool measures visual acuity and contrast sensitivity. In the simplest terms, the optokinetic reflex is a visually-evoked physiologic response induced when a visual scene crosses the retina. This elicits an eye rotation and head movement (i.e., tracking) by the animal in the direction of the image.
Location: OMRFContact: Scott Plafker,Ph.D. 405-271-1735
Odyssey CLX Infrared Imager
This infrared imager can be used for all infrared imaging of membranes, gels, culture plates and tissue sections that have been stained with infrared and near infrared robes
Location: OUHSC Contact: Ann Louise Olson Ann-olson@ouhsc.edu 405-271-1735
Nanocellect WOLF
The Nanocellect WOLF is the industry leader in microfluidic-based sorting that is compatible with mammalian, microbial, nuclei and plant-based cells. It is a simple benchtop cell sorter, with a small footprint, low PSI to protective fragile cell populations, and uses a disposable microfluidic ship with intuitive software.
Location: OSU-Stillwater Contact: Joshua.butcher@okstate.edu 405-744-8088 or madhan.subramanian@okstate.edu-405-744-3393
Cytek Aurora 4L
The Cytek Aurora uses spectral analysis to allow separation of fluorochromes with very close emission profiles. This Aurora is equipped with blue, red, yellow-green, and violet excitation lasers, with 48 fluorescence channels. Spectral flow cytometry allows for the use of a wide array of fluorochrome combinations. This instrument includes the plate sample loader with mixing and temperature control.
Location: OUHSC- DMEI Trisha McDonald, Contct: Trisha-McDonald@ouhsc.edu, (405)271-4944
 Agilent Q-TOF LC/MS System
Agilent Q-TOF LC/MS System
Quadruple Time of Flight Liquid chromatography / Mass spectrometry system designed for simultaneous wide in-scan dynamic range of small molecules, ideal for small molecule, metabolomics, lipidomics, and peptide analysis.
Location- OMRF- Contact: Tim Griffin, Ph.D. griffint@omrf.org 405-271-7579
Helios Mass Cytometer and Hyperion Imaging Mass Cytometer
In the Helios configuration, this system performs mass cytometry (also known as CyTOF) a technique that enables high-dimensional biomarker analysis on suspension cells using heavy metal labeled antibodies or probes (~40 channels possible). In the Hyperion configuration, the system performs Imaging Mass Cytometry on tissue sections to enable spatially aware analyses of both deep per-cell biomarker analyses, but also analyses of inter-cellular interactions and cell neighborhoods.
Location- OMRF- Contact: Joel Guthridge, Ph.D. ACI-Phenotyping@omrf.org 405-271-4987

Seahorse XFe96 Analyzer
The Seahorse XFe96 Analyzer is optimized for metabolic analysis of specialized cells. This analysis includes both cellular oxygen consumption and extracellular acidification (glycolysis) rates. When used with available equipment to isolate, identify, and sort cells, it can be possible to measure metabolism in as few as 5,000 unique cells per sample. This makes it possible to measure the metabolism of immune cell, adult stem cell, and progenitor cell populations. Cellular metabolism is directly related to immune cell function and cellular “stemness”. These properties affect tissue healing and regeneration. The XFe96 can test up to 92 samples in a single analysis. This large capacity allows scientists to design many different kinds of experiments. Multiple labs studying obesity and inflammation related diseases would use the XFe96.
Location- OMRF- Contact: Satoshi Matsuzaki MatsuzakiS@omrf.org 405-271-1745
MERSCOPE Platform for Spatial Transcriptomics
Vizgen’s MERSCOPE Platform is powered by the proprietary MERFISH (Multiplexed Error-Robust Fluorescence in situ Hybridization) technology, a quantitative and genome-scale multiplexed imaging technique for identifying nucleic acids in their native tissue environment with resolution scaling from subcellular to tissue scale.
Location- OMRF- Contact: Lijun Xia-lijun-xia@omrf.org-405-271-7892
The XF HS Mini analyzer is easy to set up and operate and has excellent OCR/PER precision. It is also compatible with the XF HS miniplate, which can generate robust assay signals using three times fewer cells compared to the XFp miniplate—making it good for studies when biological materials are limited.
Location- OUHSC- Contact: Kathryn Burge, Ph.D.- Kathryn-Burge@ouhsc.edu 405-271-5215 ext.47525
The Zeiss LSM 780 confocal laser scanning microscope is equipped with 4 lasers: a diode laser
(405 nm), an argon laser (458, 488, and 514 nm), a diode-pumped solid-state laser (561 nm) and
a helium-neon (633 nm). Available objectives include 10X, 40X water immersion, and 63X oil
immersion objectives, along with a highly sensitive gallium arsenide detector and two standard
photomultiplier tube detectors. The 780 is also outfitted with an environmental enclosure for maintaining temperature and CO2 levels for long-term live-cell imaging.
Location- OU-Norman. Contact: Stefan Wilhelm, Ph. D.- stefan.wilhelm@ou.edu-405-325-4982